A NEW DAWN
In Disease Early Interception
A Future Without Late Diagnosis
Harnessing RNA science to detect cancer early and save lives.
Frontiers in Discovery
Deciphering the impact of GASTROClear™
As the world’s first molecular blood test for gastric cancer, GASTROClear™ is set to play a pivotal role...
Read More 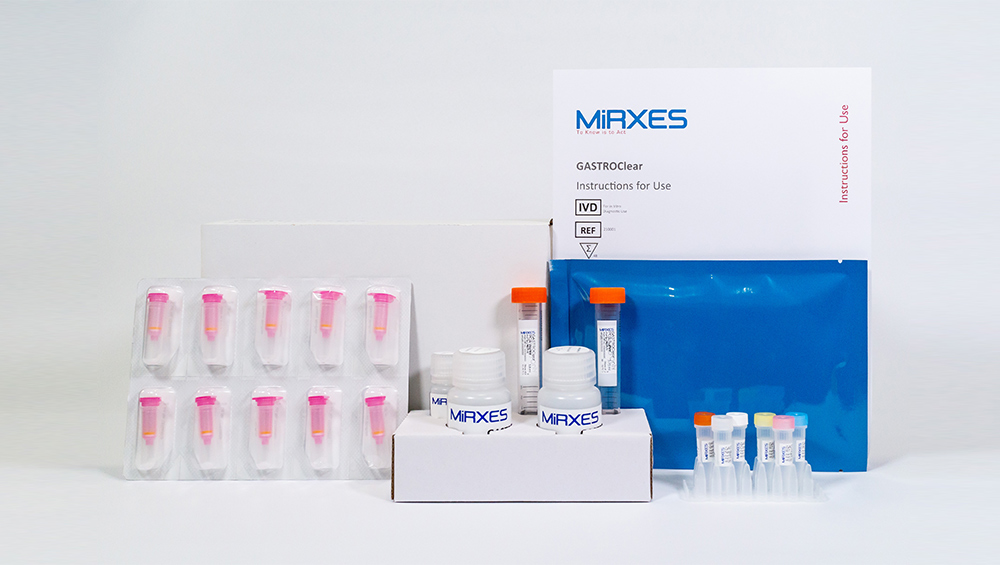
Detecting miRNAs with ID3EAL Suite
Class-leading, highly sensitive detection for miRNAs with our proprietary ID3EAL RT-qPCR technology.
Read More 
Decoding the microRNA Universe
Nobel-winning discovery of a new class of tiny RNA molecules that play a crucial role in gene regula...
Read More 
Our Technology & Solutions in the Cancer Care Continuum
Cancer Screening
GASTROClear and LUNGClear in the market, more in the pipeline.
Cancer Treatment Selection
APEX and COMPASS for targeted and comprehensive Next Generation Sequencing (NGS) respectively.
Discovery Genomics
Genomics and bioinformatics services to enhance our partners’ biological system research.
Cancer Treatment Selection
APEX and COMPASS for targeted and comprehensive Next Generation Sequencing (NGS) respectively.
Discovery Genomics
Genomics and bioinformatics services to enhance our partners’ biological system research.







