Cryomics
Partnering Discovery
About Mirxes Cryomics

Cryomics offers unmatched biobanking solutions, from secure storage to consultancy. Our state-of-the-art facility uses automation for efficient processing, storage, and management of high-quality biological samples and data. We also partner with our clients to navigate regulatory, legal, ethical, contractual and stakeholder complexities, while providing easy access to Mirxes’ suite of disease detection capabilities and Omics facilities.
Partnering at Every Step of the Journey of a Biological Sample
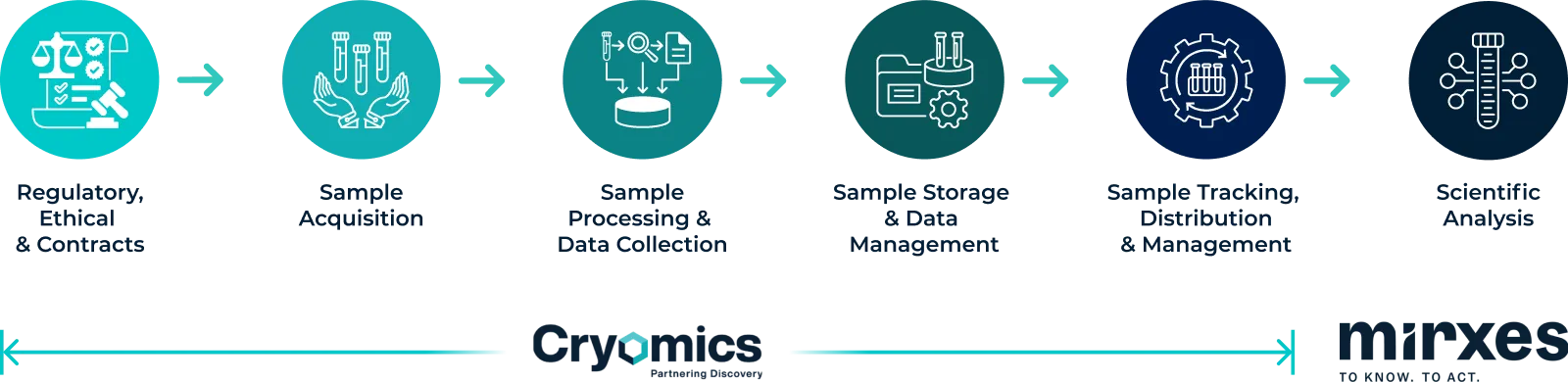














Regulatory and Compliance
Cryomics offers unparalleled regulatory and compliance expertise in biobanking. This ensures your samples meet the highest quality and integrity standards, while adhering to all relevant policies.
Regulatory Landscape
We understand the challenges posed by regulatory frameworks and ensure full compliance with applicable laws and regulations governing biobanking activities. Our experienced team guides clients through the complexities of regulatory requirements, ensuring that all necessary approvals and permissions are obtained.
Compliance
Cryomics is proud to uphold rigorous standards and compliances, adhering to the stringent regulations outlined in the 21CFR Part 11 (Code of Federal Regulations) set by the Food and Drug Administration (FDA) in the United States. Our commitment to maintaining the highest quality in biobanking practices is evident through our strict adherence to the guidelines established by the International Society for Biological and Environmental Repositories (ISBER) Best Practices. Additionally, we follow CTRNET (Canadian Tissue Repository Network) policies and standards, a testament to our dedication to ethical considerations, superior quality management, and impeccable standards in sample collection, processing, storage, and distribution.


Human Biomedical Research Act (HBRA)
Cryomics is committed to conducting human biomedical research and tissue banking activities in strict adherence to the legislation set forth by the Ministry of Health (MOH) in Singapore.
The Human Biomedical Research Act (HBRA) provides clarity regarding the roles and responsibilities of individuals and body corporates involved in human biomedical research and the handling of human tissue for use in research, emphasizing ethics and scientific standards to safeguard the safety and well-being of both research subjects and tissue donors.


Personal Data Protection Act (PDPA)
Cryomics complies with the guidelines and regulations outlined in The Personal Data Protection Act (PDPA) which establishes a fundamental level of safeguarding for personal data in Singapore. PDPA encompasses diverse mandates that oversee the acquisition, utilization, disclosure, and maintenance of personal data within the country.


General Data Protection Regulation (GDPR)
Cryomics adheres to GDPR and ensures compliance by obtaining explicit consent for data processing, implementing robust security measures for data protection, and maintaining transparent privacy policies.
GDPR, or General Data Protection Regulation, is a comprehensive data privacy law that protects the personal information of EU citizens. It applies to organizations
worldwide that handle EU residents’ data and imposes strict requirements for data processing, storage, and protection. GDPR gives individuals greater control over their data, including the right to access, correct, and delete their information. Non compliance can lead to substantial fines, making GDPR compliance crucial for businesses and institutions. The regulation aims to strengthen data privacy rights and promote trust in the digital economy.
Governance, Contracts & Ethics
Cryomics combines expertise in governance, contractual matters, and ethical considerations to offer comprehensive biobanking services, ensuring the highest standards of quality, integrity, and adherence to best management practices and legal and ethical frameworks.
Our operations are underpinned by the pivotal role of biobanking governance in ensuring smooth day-to-day functionality. Given the paramount significance of biospecimens, we prioritize the implementation of robust business and continuity planning alongside meticulous legacy planning.
Contracts are an integral part of biobanking collaborations, and we assist our clients in navigating the contractual landscape. From drafting agreements to negotiating terms, we provide guidance to establish clear obligations and responsibilities between all involved parties. Our cooperative approach to stakeholder management promotes collaboration and mutual understanding.
Ethical considerations play a crucial role in biobanking, and we are committed to upholding the highest ethical standards. We prioritize informed consent, privacy protection, and the responsible use of collected samples and data. Our comprehensive strategies encompass ethical review processes, confidentiality safeguards, and transparent practices to ensure the well-being and rights of donors and participants.
Speak to our Biobanking Experts






Biobanking
Biobanking involves the systematic collection, storage, and management of biological samples and associated data. It serves as a vital resource for research, enabling scientists to study diseases, genetics, and personalized medicine. Ethical considerations, such as informed consent and data privacy, are integral to biobanking practices. Ultimately, biobanks play a pivotal role in advancing medical knowledge and developing innovative treatments.

Bio-Sample Processing and Data Collection
Cryomics excels in sample processing and data collection by employing state-of-the-art technologies, implementing stringent quality control measures, and prioritizing data privacy and security. Our expertise ensures the reliability, accuracy, and integrity of both the processed samples and the associated data, making us a trusted partner in biobanking.
Bio-Sample Processing
Our state-of-the-art facility is equipped with cutting-edge technology to handle various types of biological samples. We have standardized protocols and quality control measures in place to maintain the integrity and viability of the samples throughout the processing workflow. Whether it’s DNA, RNA, proteins, or other biomolecules, our skilled technicians employ meticulous techniques to extract, isolate, and prepare samples for downstream analysis.
Data Collection
We understand the significance of comprehensive and high-quality data in biobanking. We implement robust data collection strategies to capture relevant information associated with each sample. This includes detailed clinical annotations, demographic data, medical history, and any other pertinent information. We prioritize data accuracy, ensuring precise documentation and adherence to data privacy regulations.
Quality Assurance
Our biobank maintains rigorous quality assurance practices throughout the sample processing and data collection processes. We have implemented standardized operating procedures, quality control checks, and validation protocols in line with ISBER best practices to ensure the reliability and consistency of our results. Our quality assurance measures guarantee that the collected samples and associated data meet the highest standards of accuracy and integrity.

Bio-Sample Storage & Data Management
Cryomics offers bio sample storage services that prioritize optimal conditions, meticulous tracking, stringent security measures, and compliance with regulatory guidelines. With our advanced facilities and expertise, we ensure the long-term preservation and accessibility of valuable biological samples in biobanking.
Bio-State-of-the-Art Facilities
We take pride in our state-of-the-art storage facilities specifically designed to cater to the unique requirements of biological samples. Our facilities are equipped with advanced temperature and humidity control systems to maintain optimal storage conditions for different types of samples. We strictly adhere to industry best practices and regulatory guidelines to ensure sample stability and viability over extended periods.
Temperature Control
Cryomics understands the critical role temperature plays in sample preservation. We offer storage solutions with a range of temperature options, including ultra-low temperatures (e.g., -80°C or lower), liquid nitrogen conditions (-196°C), standard freezer temperatures (e.g., -20°C), and refrigerated conditions. This allows us to accommodate diverse sample types and storage needs while minimizing the risk of degradation or loss of sample quality.
Integration of Technologies
We leverage cutting-edge technologies for seamless integration of sample processing and data collection. We utilize advanced laboratory automation systems and specimen inventory management systems to streamline workflows, minimize errors, and enhance efficiency. These technologies enable efficient tracking and management of samples and associated data, ensuring a smooth and traceable process.
Data Privacy and Security
We prioritize data privacy and security. We employ robust data management strategies, including strict access controls, encryption, and compliance with relevant data protection regulations. Confidentiality and privacy safeguards are in place to protect the sensitive information associated with the samples and participants.


Bio-Sample Tracking, Distribution & Management
Comprehensive Sample Tracking
We employ robust sample tracking systems to monitor and manage samples throughout their storage journey. Each sample is assigned a unique identifier and meticulously tracked using a Specimen Inventory Management System. This ensures accurate and efficient retrieval of samples when needed and provides a secure audit trail of their handling and storage history.
Security and Access Control
We prioritize sample security and maintains strict access control measures. Our facilities are equipped with advanced security systems, including restricted access areas, surveillance cameras, and alarms. Only authorized personnel are granted access to the storage areas, minimizing the risk of unauthorized handling, or tampering.
Backup and Disaster Recovery
We implement comprehensive backup and disaster recovery strategies to safeguard against unforeseen events. This includes redundant storage systems, off-site backups, and contingency plans to protect samples in the event of power outages, equipment failures, or natural disasters.
Compliance and Documentation
We adhere to regulatory and industry standards to ensure compliance with relevant guidelines for sample storage. Our processes include thorough documentation of storage conditions, handling protocols, and associated metadata, ensuring transparency and traceability for regulatory audits and research integrity.

Cryomics works together with Mirxes to offer a wide array of scientific analysis services downstream of biobanking, encompassing molecular profiling, proteomics, metabolomics, histopathology, imaging, omics data integration, and collaborative research. With our advanced technologies and skilled experts, we strive to unlock the potential of stored biological samples and provide valuable scientific insights for research, diagnostics, and therapeutic development.
Research
Diagnostics
Diagnostic Kits
Omics
Treatment Development
Work With Us
We offer a comprehensive portfolio of services within the field of biobanking and scientific research. Our services portfolio includes the following:
- Sample Collection and Processing: Expert handling and processing of biological samples, ensuring sample integrity and quality preservation.
- Sample Storage: State-of-the-art storage facilities with temperature-controlled options, ensuring optimal conditions for various sample types.
- Sample Management: Comprehensive sample tracking, inventory management, and sample retrieval services.
- Sample Shipping: Safe and efficient transportation of samples with adherence to regulatory requirements.
- Regulatory Compliance Guidance: Assistance in navigating regulatory frameworks and obtaining necessary approvals.
- Governance: Support in business continuity planning, legacy planning and overall best operational and management practices.
- Ethical Considerations: Adherence to ethical guidelines, informed consent protocols, and privacy protection measures.
- Documentation and Audit Support: Comprehensive documentation of processes, protocols, and regulatory compliance measures for audits and inspections.
- Stakeholders Identification: Relevant stakeholders including researchers, clinicians, healthcare professionals, regulatory bodies, ethics committees, funding agencies, and participants/donors.
- Stakeholder Analysis: Understanding of stakeholders roles, interests, expectations, and potential impact on the biobanking project or service.
- Stakeholder Management: Understanding and meeting the needs and expectations of different stakeholders.
- Workshops and Training: Provision of workshops, training sessions, and educational resources on biobanking best practices, regulatory compliance, and scientific analysis.
Get in Touch
Cryomics understands that each customer and research project is unique, which is why we offer customized solutions tailored to meet specific needs.
For our customized solution offerings please fill the form.
Contact Us
#08-01 JTC Medtech Hub 2,
Tukang Innovation Grove,
Singapore 618305
Email: cryomics-info@mirxes.com