ELEGANCE Study
EarLy DEtection of HCC: miRNA, microbiome and imaGing biomArkers in the evolution of chroNiC livEr Disease
ELEGANCE Study
EarLy DEtection of HCC: miRNA, microbiome and imaGing biomArkers in the evolution of chroNiC livEr Disease
A Whole-of-Nation Effort in Early HCC Liver Cancer Detection
Launched in 2021, ELEGANCE Study is a pioneering cohort clinical study aimed at advancing the early diagnosis and prediction of hepatocellular carcinoma (HCC), the primary type of liver cancer. HCC is the sixth most common cancer in the world but the third most common cause of cancer deaths globally [1]. In Singapore, it is the third most common cause of cancer death in men and fifth in women [2].
This multi-centre study, led by the National Cancer Centre of Singapore (NCCS), involves a nationwide collaboration with leading healthcare institutions and academic partners, including Singapore General Hospital (SGH), National University Hospital (NUH), Changi General Hospital (CGH), Sengkang General Hospital (SKH), Tan Tock Seng Hospital (TTSH), eight SingHealth Polyclinics, Duke-NUS Medical School and Singapore Phenome Centre (Lee Kong Chian School of Medicine).
By leveraging the expertise of clinician-scientists, researchers and cutting-edge technology from both the public and private sectors, ELEGANCE Study seeks to develop more accurate diagnostic tools and identify potential therapeutic targets for HCC. The clinical study aims to enrol 2,000 participants, including patients with liver cirrhosis, hepatitis B or C, and fatty liver, a group deemed to be at high risk of developing HCC
References
- Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424.
- National Registry of Diseases Office. (2023, Aug). Singapore Cancer Registry Annual Report 2021








Mirxes' Involvement and Contribution
Mirxes plays a pivotal role in one of the three key parts of ELEGANCE Study as an industry partner. By harnessing Mirxes’ cutting-edge technology and expertise in microRNAs (miRNAs), NCCS and Mirxes seek to develop and evaluate a miRNA diagnostic kit for detection of liver cirrhosis from patient blood samples.
It was announced in May 2024 that initial analyses have led to the discovery of a miRNA biomarker panel for liver cirrhosis. Further validation through laboratory and clinical trials is now being conducted, together with commercialisation consideration.

Liver Cirrhosis and Liver Cancer
Liver cirrhosis is a severe condition where the liver becomes progressively scarred and hardened, hindering its function. This irreversible damage can lead to liver failure, increased risk of liver cancer, and ultimately, death. Early stages of liver cirrhosis often go unnoticed due to a lack of symptoms.
Late stage scarring of liver tissue (cirrhosis)
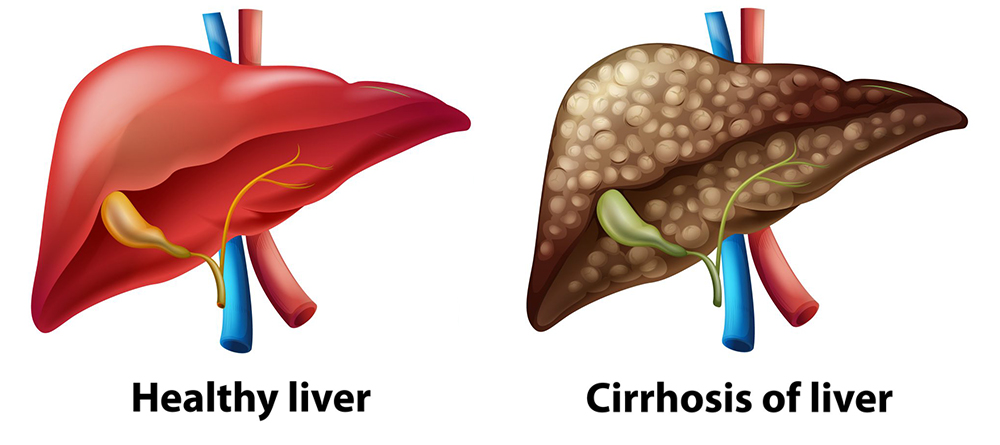
Photo credit: Freepik
Potential Impact on Global Health
Patients with liver cirrhosis and HCC often display no symptoms in the early stages. Current diagnostic methods, which include imaging and blood-based tests, lack accuracy and may not detect early-stage disease. On the other hand, liver biopsy, the most definitive diagnosis for both liver cirrhosis and HCC, is an invasive procedure with potential risks, including pain and internal bleeding.
The development of the miRNA-based biomarker panel offers a promising solution for wider accessibility, more precise tracking of disease progression and severity, and most importantly, timely intervention for improved patient outcomes. Currently, only 20% of HCC cases are detected at a stage where a cure is possible.
LB-1349_r00

Photo credit: Freepik

SOCIAL MEDIA
- Genomics
- Clinical Sequencing
- Research Sequencing
- Cancer Screening
- GASTROClear for Patients
- GASTROClear for Medical Professionals
- Cancer Treatment Selection
- APEX Tissue Panel for Medical Professionals
- Infectious Diseases
- Fortitude 2.1
- Fortitude 3.0
- Fortitude Syndromic Panel
- CoVClear Mutation Panel
- Swab & Saliva Collection
- RNA Extraction Kit & Instruments

SOCIAL MEDIA
- Genomics
- Clinical Sequencing
- Discovery Genomics
- Cancer Screening
- GASTROClear for Patients
- GASTROClear for Medical Professionals
- LUNGClear
- Cancer Treatment Selection
- APEX Tissue
- COMPASS Tissue
- Infectious Diseases
- Fortitude 2.1
- Fortitude 3.0
- Fortitude Syndromic Panel
- CoVClear Mutation Panel
- Swab & Saliva Collection
- RNA Extraction Kit & Instruments